 |
| The Clinical Trials Research Centre is investigating a new HPV vaccine. |
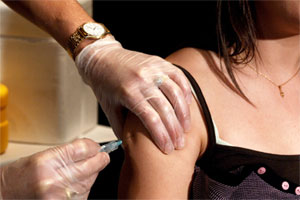
Researchers at the Clinical Trials Research Centre are seeking young women to participate in a study testing a new HPV vaccine. More than 150 women between the ages of 16 and 26 are needed for the four-year study.
The human papillomavirus (HPV) is a common virus transmitted through genital skin-to-skin contact. Seventy-five per cent of Canadians will have at least one HPV infection in their lifetime. Most never know they have been infected. The virus can lead to warts, abnormal pap tests, or even cervical cancer.
Vaccination to prevent HPV infection is the best way to prevent genital warts and cervical cancer. The only available vaccine that protects against cervical cancer is Gardasil, approved by Health Canada in July 2006. Gardasil protects against four of the most common types of HPVâtypes 6, 11, 16 and 18. HPV types 16 and 18 are high-risk types and cause approximately 70 per cent of cervical cancers. HPV types 6 and 11 are low-risk types and cause approximately 90 per cent of external genital warts.
Like Gardasil, the new vaccine being studied is from the pharmaceutical company Merck. But this vaccine may be able to protect against five more strains of HPV, âpotentially providing protection against an additional 10 per cent of cervical cancers,â says Dr. Shelly McNeil, an Infectious Diseases specialist at the Queen Elizabeth II Health Sciences Centre and vaccine researcher with the Clinical Trials Research Centre.
âThe women who participate will be randomized to receive either Gardasil or the new study vaccine,â adds Dr. McNeil. âNo one will receive a placebo; they would at least receive Gardasil.â
In 2007-08 academic year, the provincial government set up a publicly funded program for girls in Grade 7 to receive three doses of the HPV vaccine over a six-month period. Starting this fall, the HPV vaccine will also be offered to female students in Grade 10.
Outside of the provincial program, women who would like to receive the vaccine have to pay for it themselves. But itâs costly: the three doses of the vaccine cost between $400 and $500. However, by participating in the study, participants will receive the vaccine for free.
âThe important issue with this trial is its long duration; we will be following volunteers for four years,â says Dr. McNeil.
The first year of the study is the busiest, requiring six visits to the Clinical Trials Research Centre, says research coordinator Robyn McCall-Sani. In the subsequent three years, volunteers will need to visit the centre, located on the fourth floor of the Goldbloom Pavillion at the IWK Childrenâs Centre, for twice-a-year pap tests.
Women who are interested in participating in the study should contact Ms. McCall-Sani by phoning 470-7839 or by email at robyn.sani@iwk.nshealth.ca.
The Clinical Trials Research Centre is a division of the Canadian Center for Vaccinology, a multidisciplinary research group based at ОÓÆÂÁųšÏēĘŋŠ―ąÖąēĨ, the IWK Health Centre, and Capital Health.
